Feature
STORAGE INSTRUCTIONS:
1. Store at room temperature (2-30ºC or 35.6-86°F) in a dry place. Avoid direct sunlight;
2. 18 months of shelf life (date of manufacture to expiration date).
Specimen collection
A) Nasal Aspiration
Collect nasal aspirate fluids using the specific aspirate as instructed.
B) Nasal Swabbing
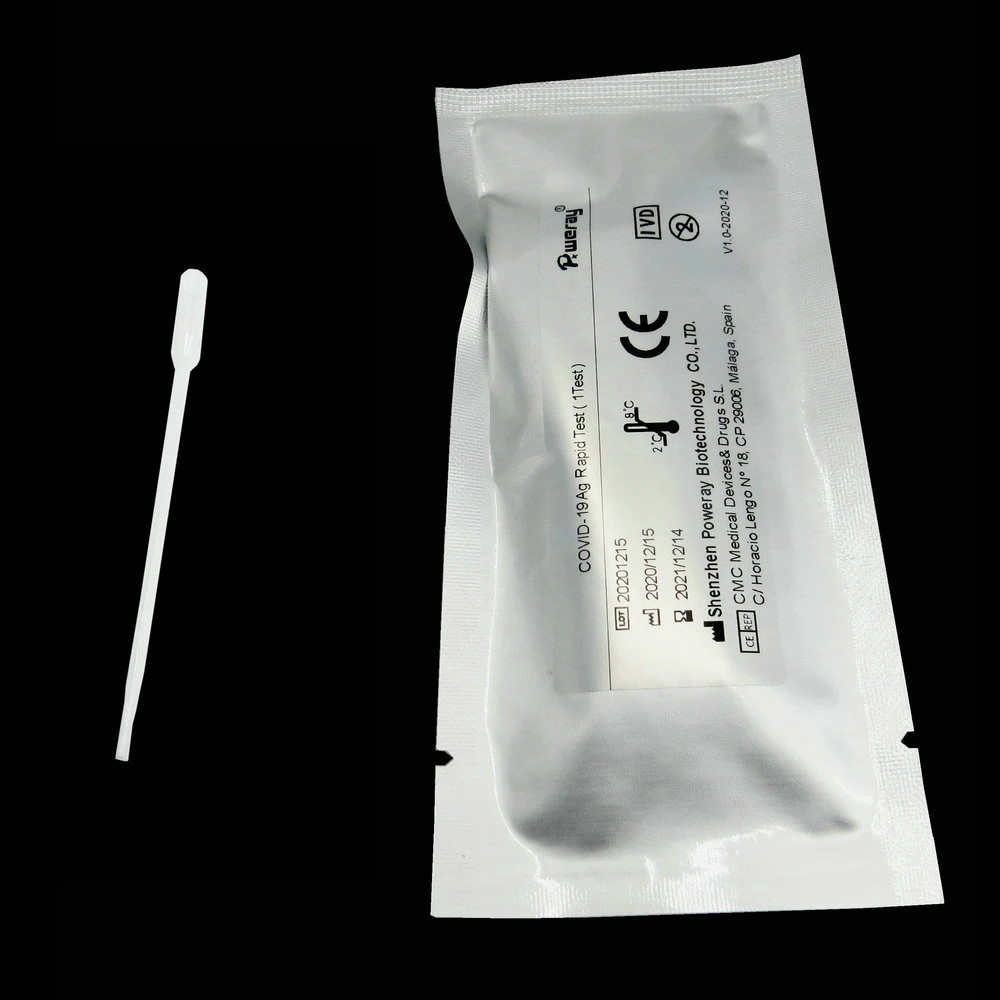
Completely insert the sterilized swab, which is supplied in this kit into the nasal basin, and swab
several times to collect the epidermal cells of mucus.
C) Throat Swabbing
Insert the sterilized swab deep into the throat and swab several times to collect the epidermal cells of the
mucus. Contamination of the swab with saliva should be avoided.
Specimen extraction
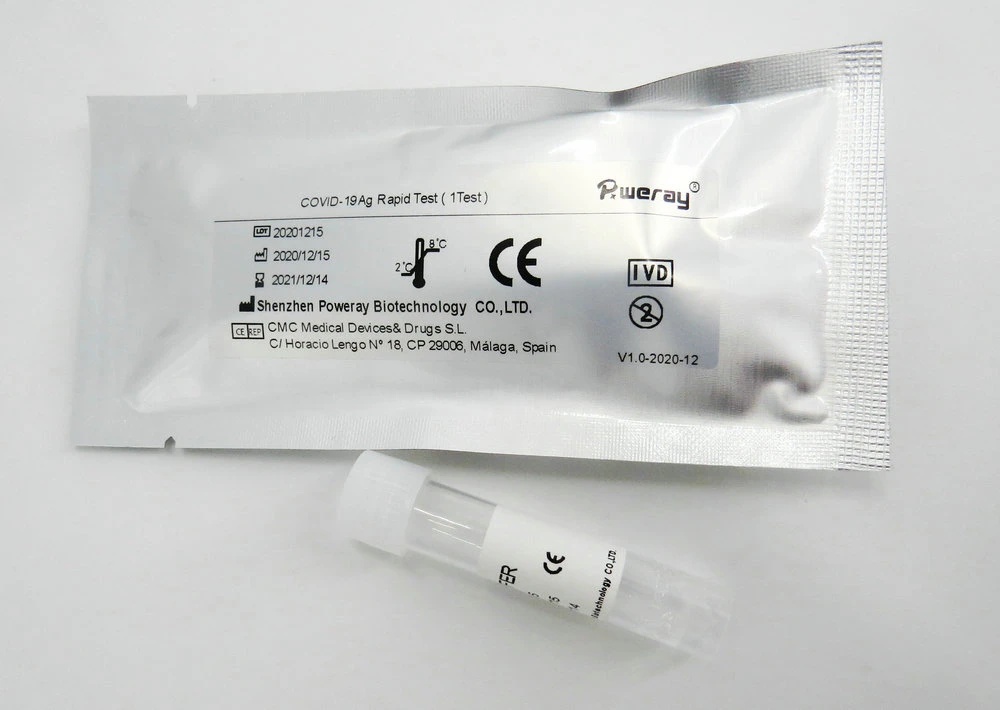
Add 0.5 ml or 0.5-1.0 ml of the extraction solution into an extraction tube which is supplied in this kit,
and put it on the stand.
A) Nasal Wash/Aspirate
Add 0.5ml of the nasal aspirate fluid to 2ml of transfer buffer or physiological saline. Then add 0.5ml of
this mixture to the extraction tube which contains 0.5 ml of the extraction solution, and mix well by
squeezing. The extraction mixture will be used as test sample.
B) Nasal or Throat swabs
Place the swab used to collect the sample into an extraction tube which contains 0.5-1.0 ml of the
extraction solution, and immerse the swab by squeezing the tube several times. Remove the swab and use
the extraction mixture as test sample.
TESTPROCEDURE:
Step 1 Bring the test to room temperature (18-26ºC) before use.
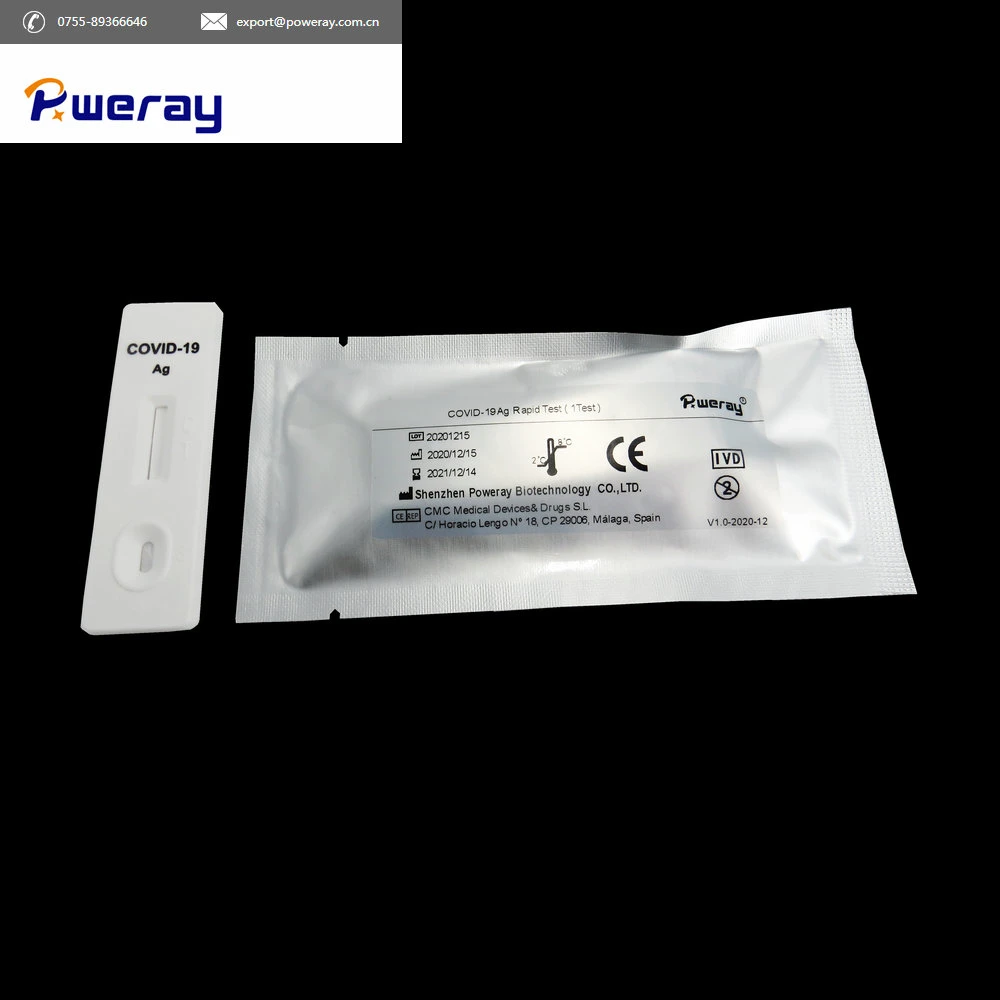
Step 2 Open the foil pouch, take out the test and lay it on an even surface.
Step 3 Add 3 drops(about 100 µL )of extraction mixture into the each sample well (S).
Step 4 Read and record the results at 15 minutes after addition of samples.
1. Store at room temperature (2-30ºC or 35.6-86°F) in a dry place. Avoid direct sunlight;
2. 18 months of shelf life (date of manufacture to expiration date).
Specimen collection
A) Nasal Aspiration
Collect nasal aspirate fluids using the specific aspirate as instructed.
B) Nasal Swabbing
Completely insert the sterilized swab, which is supplied in this kit into the nasal basin, and swab
several times to collect the epidermal cells of mucus.
C) Throat Swabbing
Insert the sterilized swab deep into the throat and swab several times to collect the epidermal cells of the
mucus. Contamination of the swab with saliva should be avoided.
Specimen extraction
Add 0.5 ml or 0.5-1.0 ml of the extraction solution into an extraction tube which is supplied in this kit,
and put it on the stand.
A) Nasal Wash/Aspirate
Add 0.5ml of the nasal aspirate fluid to 2ml of transfer buffer or physiological saline. Then add 0.5ml of
this mixture to the extraction tube which contains 0.5 ml of the extraction solution, and mix well by
squeezing. The extraction mixture will be used as test sample.
B) Nasal or Throat swabs
Place the swab used to collect the sample into an extraction tube which contains 0.5-1.0 ml of the
extraction solution, and immerse the swab by squeezing the tube several times. Remove the swab and use
the extraction mixture as test sample.
TESTPROCEDURE:
Step 1 Bring the test to room temperature (18-26ºC) before use.
Step 2 Open the foil pouch, take out the test and lay it on an even surface.
Step 3 Add 3 drops(about 100 µL )of extraction mixture into the each sample well (S).
Step 4 Read and record the results at 15 minutes after addition of samples.
| Products name | Nucleic Acid Detection Kit One Step Influenza Flu 19 Antigen Antibody IgG IgM Rapid Test Kit |
| Stick material | Plastic |
| Material | ABS |
| Color | Picture shown |
| usage | Flu 19 Antigen Antibody IgG IgM Rapid Test |
| Product Description | Nucleic Acid Detection Kit |
| Packing specifications | 1 Test Package |
| Note | Storage 2-30ºC |
For Nucleic Acid Detection Rapid Test Ag
1. Negative reference coincidence rate Use 10 samples of Flu 19 Ag negative reference to test, the test results should not be positive, the negative coincidence rate should be 10/10;
2. Positive reference coincidence rate Use 10 samples of Flu 19 Ag positive reference to test, the test result should all be positive, the positive coincidence rate should be 8/10;
3. Sensitivity Use 3 samples of Flu 19 Ag sensitivity reference to test, L1 should be detected, L2 should be detected or not, L3 should not be detected.
4. Repeatability 5 / 6 Use 1 sample of internal control repeatability reference( J1) to complete the parallel test for 10 tests, the results should be coincident.
5. Cross-reaction and interference reaction Use positive samples of Rhinovirus, RS virus, Influenza A virus, Influenza B virus, Chlamydia, Mycoplasma and Bacterial Infection to do the tests, the test results should be negative.
6. The Clinical study The Flu 19 Ag Rapid Test has been evaluated with 155 clinical samples. Both 91 negatives and 64 positives were confirmed by PCR
NOTE:
1. The intensity of color in the test line (T) may vary depending on the concentration of analyses present
in the specimen. Therefore, any shade of color in the test line (T) should be considered positive. Please
note that this is a qualitative test only, and cannot determine the concentration of analyses in the
specimen.
2. Insufficient specimen volume, incorrect operating procedure or expired tests are the most likely
reasons for control band failure.
QUALITY CONTROL:
It is recommended to use control samples. The use of control samples is advised to assure the day to day
validity of results. Use controls at both normal and pathological levels.
It is also recommended to make use of national or international Quality Assessment programs in order to
ensure the accuracy of the results.
Employ appropriate statistical methods for analyzing control values and trends. If the results of the assay
do not fit to established acceptable ranges of control materials patients results should be considered
invalid. In this case, please check the following technical areas: Pipetting and timing devices; expiration
dates of reagents, the specimen collection procedure and storage conditions.


 FAQ:
FAQ:Q1: Why choose us?
Poweray is located in Shenzhen Pingshan National Bioengineering Base. Both OEM and ODM are available besides sampling tube products, L/T is accurate and same quality control standard per sample confirmed.
Q2: Order processing
MOQ and customized factors confirmed. Exporting and Importing certificates verified. Firm shipping schedule on deposit with PO.
Q3: VTM Specimen Collection
Wearing medical protective clothing and gloves to keep sterile operation is important and prerequisite. Following standard sampling kits setting and operating instructions to collect good nas safely. Washing gloves put on the hands if necessary during collection schedule.