[Packaging Specifications]
20 persons/box
[Intended Use]
The product is used for qualitative detection of IgM/IgG in human serum and plasma samples in vitro. It is suitable for auxiliary diagnosis of novel infection.
[Main components]
[Sample requirements]
Serum and plasma can be used for testing.
Serum/plasma sample collection: Serum and plasma should be separated as soon as possible after blood collection to avoid hemolysis.The separated serum and plasma should be tested as soon as possible. If they can not be used in time, they should be stored at 2 ~ 8 ºC, and frozen at - 20 ºC for more than 3 days. Attention should be paid to restore to room temperature before testing to avoid repeated freezing and thawing.Samples with severe hemolysis and heat inactivation are not recommended.
[Detection Method]
1. Preparation:
(1) The test card, the sample diluent and the tested sample are restored to room temperature.
(2)Make the corresponding number on the test card according to the sample name or code.
2. Sample addition:
(1)Serum/plasma sample: Take 1 drop of serum/plasma sample with a pipette and add 2 to 3 drops of sample diluent vertically to the sample well.
(2)After adding samples, positive samples can be detected within 5 to 10 minutes.After experimental verification, reaction time more than 10 minutes may have an impact on the test results, so it is recommended to observe the test results within 10 minutes.
[Interpretation of monitoring results]
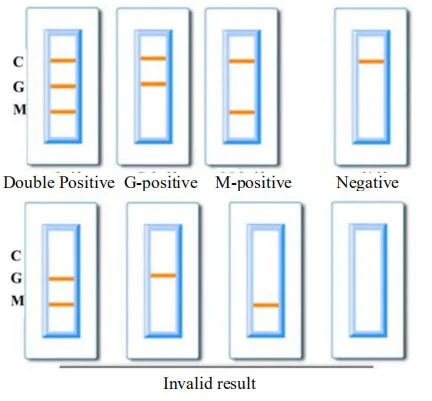
1. Positive results: Control line (C) and detection line (G and M, G or M) appear.
2. Negative results: only one control line (C) appeared, no detection lines (G and M) appeared.
3.Invalid result: No line or only test line (G and M, G or M) appears, indicating that the test is invalid, please retest the sample.

ShengShi Dotop Jiangsu Biological Technology Co.,Ltd. founded in May 2014, registered capital of RMB 20 million, is a visit to the United States, postdoctoral scholars as the core of excellent research and development team of science and technology enterprises. The workshop of 5000M2 is designed and built according to GMP standard, with the introduction of advanced equipment and production technology. After more than five years of development, it has gradually become a medical device industrial company integrating technology R&D, production and manufacturing, terminal sales and service support.
The company has passed CE certification to export to Asia, America, Africa, Middle East and other countries all over the world.


20 persons/box
[Intended Use]
The product is used for qualitative detection of IgM/IgG in human serum and plasma samples in vitro. It is suitable for auxiliary diagnosis of novel infection.
[Main components]
| Serial Number | Constituent | Packing |
| Specification | ||
| 1 | New virus IgM/IgG Test Card 20 persons | 20 persons/box |
| 2 | Straw | 20 branches |
| 3 | Sample diluent | 4ml × 1 bottle |
| 4 | Instructions | 1 copy |
| 5 | Qualification Certificate | 1 |
[Sample requirements]
Serum and plasma can be used for testing.
Serum/plasma sample collection: Serum and plasma should be separated as soon as possible after blood collection to avoid hemolysis.The separated serum and plasma should be tested as soon as possible. If they can not be used in time, they should be stored at 2 ~ 8 ºC, and frozen at - 20 ºC for more than 3 days. Attention should be paid to restore to room temperature before testing to avoid repeated freezing and thawing.Samples with severe hemolysis and heat inactivation are not recommended.
[Detection Method]
1. Preparation:
(1) The test card, the sample diluent and the tested sample are restored to room temperature.
(2)Make the corresponding number on the test card according to the sample name or code.
2. Sample addition:
(1)Serum/plasma sample: Take 1 drop of serum/plasma sample with a pipette and add 2 to 3 drops of sample diluent vertically to the sample well.
(2)After adding samples, positive samples can be detected within 5 to 10 minutes.After experimental verification, reaction time more than 10 minutes may have an impact on the test results, so it is recommended to observe the test results within 10 minutes.
[Interpretation of monitoring results]

1. Positive results: Control line (C) and detection line (G and M, G or M) appear.
2. Negative results: only one control line (C) appeared, no detection lines (G and M) appeared.
3.Invalid result: No line or only test line (G and M, G or M) appears, indicating that the test is invalid, please retest the sample.

ShengShi Dotop Jiangsu Biological Technology Co.,Ltd. founded in May 2014, registered capital of RMB 20 million, is a visit to the United States, postdoctoral scholars as the core of excellent research and development team of science and technology enterprises. The workshop of 5000M2 is designed and built according to GMP standard, with the introduction of advanced equipment and production technology. After more than five years of development, it has gradually become a medical device industrial company integrating technology R&D, production and manufacturing, terminal sales and service support.
The company has passed CE certification to export to Asia, America, Africa, Middle East and other countries all over the world.


