Nucleocapsid (N) protein is the most abundant protein with highly conserved in Co*I*G-19. N protein is used as the core raw material of rapid diagnostic reagent for immunology in the market.
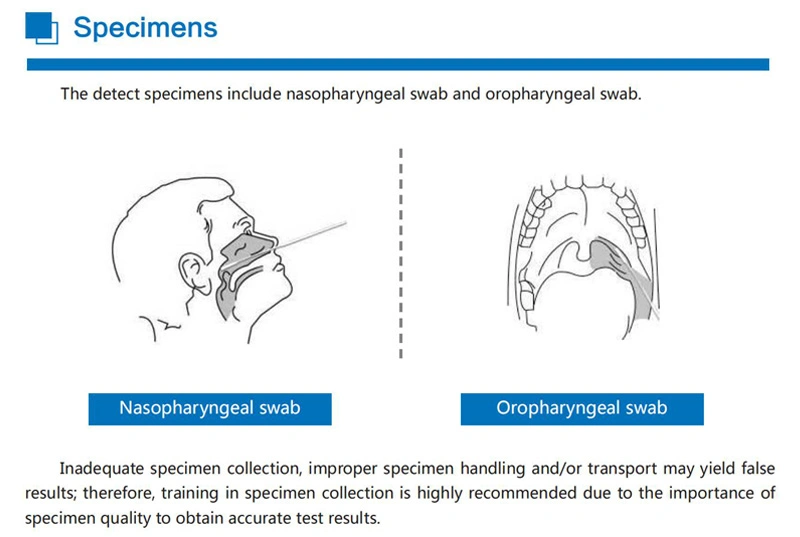
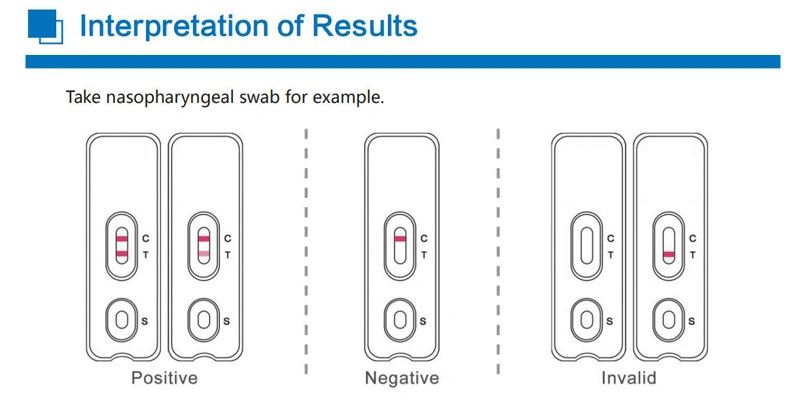
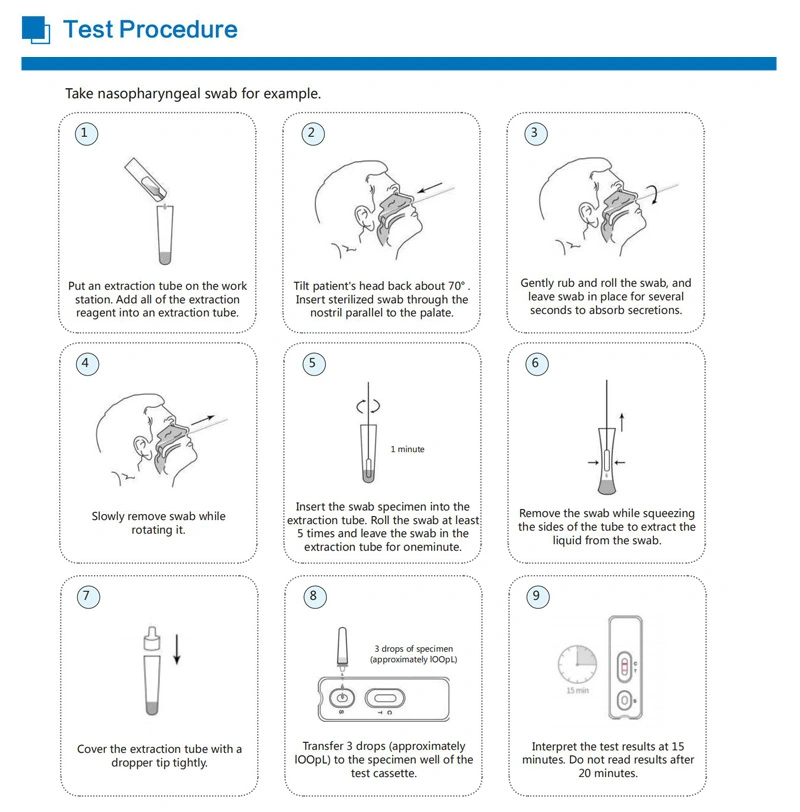
Superbio flow immunoassay intended for the qualitative detection CoV** nucleocapsid antigens in nasopharyngeal swab and oropharyngeal swab from individuals who are suspected of C*V**-19 by their healthcare provider.

CO*ING-19 Antigen | RT-PCR | Total | ||
Positive | Negative | |||
| Superbio | Positive | 64 | 0 | 64 |
| Negative | 6 | 215 | 221 | |
| Total | 70 | 215 | 285 | |



Q1. When can I get the quotation?
We usually quote within 24 hours after we get your inquiry. If you are urgent to get the price, please send the message on trade
management or call us directly.
Q2. How can I get a sample to check your quality?
After price confirmed, you can require for samples to check quality.
If you need the samples, we will charge for the sample cost. But the sample cost can be refundable when your quantity of first
order is above the MOQ
Q3. Can you do OEM for us?
Yes, the product packing can be designed as you want.
Q4. What is the MOQ of your products?
Different products and types have different MOQ, but all the goods, samples order are available.
Q5. How about your company prepare goods time?
We have stock for most daily sales products, normal prepare time is about 7 workdays. If the order products need customized
service, the prepare time will decided to the order quantity.