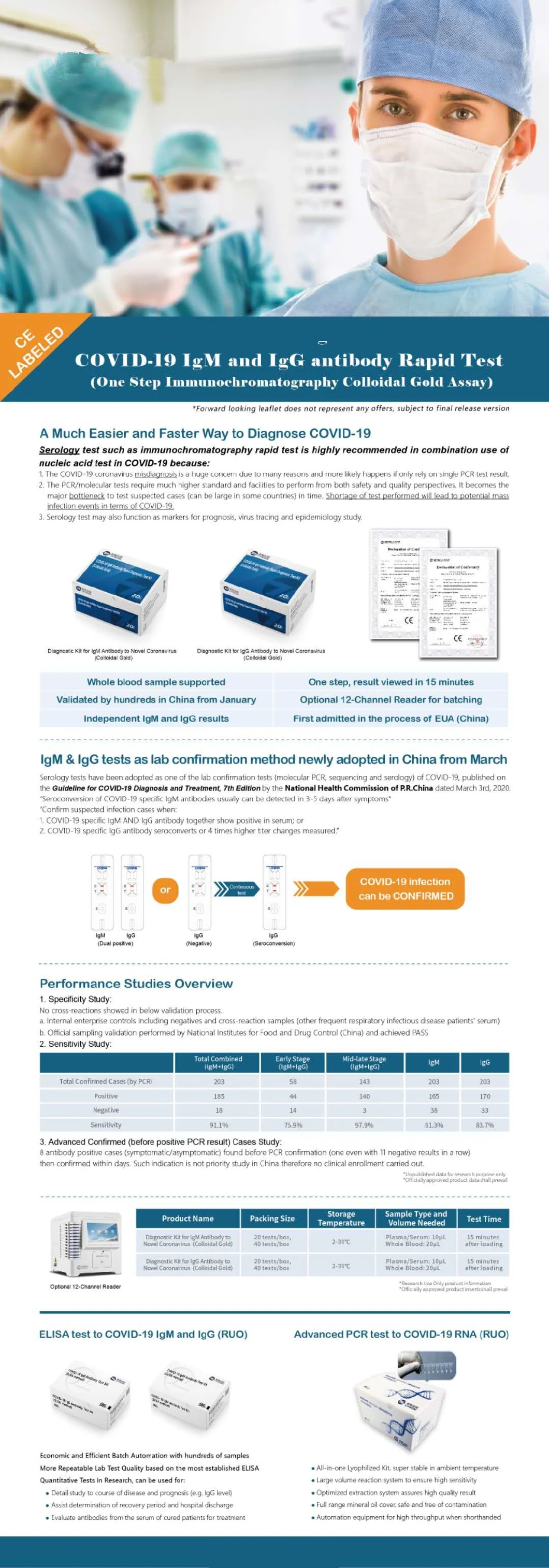
1. Specificity Study:
No cross-reactions showed in below validation process.
a. Internal enterprise controls including negatives and cross-reaction samples ( other frequent respiratory infectious disease patients'serum)
b. Official sampling validation performed by National Industries for Food and Drung Control (China) and achieved PASS.
2. Sensitivity Study:
Total Combined (IgM+igG) | Early Stage (IgM+IgG) | Mid-late Stage (IgM+IgG) | IgM | IgG | |
Total Confirmed Cases(by PCR) | 203 | 58 | 143 | 203 | 203 |
Positive | 185 | 44 | 140 | 165 | 170 |
Negative | 18 | 14 | 3 | 38 | 33 |
Sensitivity | 91.1% | 75.9% | 97.9% | 81.3% | 83.7% |
8 antibody postive cases ( sympomatic/asymptomatic) found before PCR confirmation ( one even with 11 negative results in a row) then confirmed within days. Such indication is not priority study in China therefore no clinical enrollment carried out.