The product has passed the NMPA(China National Medical Products Administraction) authority certification.
Basic Info.
| Product | Nucleic Acid detection kit (Real-Time Fluorescent RT-PCR) | Storage Temperature | -20±5ºC |
| Transport Package | Carton Package with Full Ice Storage | Shelf Life | 6 months |
| Application | Diagnostic, Laboratory, Hospital, CDC | Package Weight | 70KG |
| Package Spec. | 100 tests/kit. | Package Size | 641*552*743mm |
| 50 tests/kit. | |||
| 25 tests/kit | |||
| Origin | China | Certification | CE/NMPA/TGA |
| Lead Time | 3~5 working days | Port | Shenzhen |
How C0r0navlrus Tests Work
1)Sample Collection
The first step in any test is collecting a sample. Doing so involves placing a sterile swab at the back of a patient's nasal passage, where it connects to the throat via the nasopharynx, for several seconds to absorb secretions. After a sample is collected, the swab goes into a liquid-filled tube for transport.2)Viral Detection
To determine whether a nasopharyngeal sample is positive for the, biotechnicians use a technique known as reverse transcriptase polymerase chain reaction, or RT-PCR. The World Health Organization's and CDC's test kits both use this method, as do all of the kits the latter has approved to date.
There's a lot of hands-on work involve in performing RT-PCR tests. First, a technician extracts viral genetic material called RNA-if it is present-from the sample and uses it to produce a complimentary strand of DNA that the RT-PCR technique amplifies, or makes thousands of copies of, to get a measurable result.
And there is a risk of a false-negative result if the sample is not taken correctly.This possibility could explain why people recovering from the disease sometimes test negative initially and then positive later.
This test kit had been sold and widely used in China as well as Morocco, Italy, Indonesia, the USA etc.
Unlike rapid test, the Nucleic Acid test kit(Real-Time Fluorescent RT-PCR) is intended for use in the lab by trained personnel. With high sensitivity and specificity, nucleic acid test is suggested by worldwide professionals and experts for confirmatory diagnosis of infection.
[Product Name] Nucleic Acid detection kit( Real-Time Fluorescent RT-PCR)

For in vitro diagnostic use only. For professional use only
| Intended for use | Qualitative detection of nucleic acid in upper and lower respiratory tract specimens. |
| Secimens Type | oropharyngeal swabs, nasopharyngeal swabs and sputum. |
| Methodology | Fluorescence PCR. |
| Design of Target | ORF1ab and N gene |
| Package Spec. | 25 tests/kit;50 tests/kit;100 tests/kit. |
| Main components | Reaction fluid;RT-PCR enzyme; Negative&Positive quality controls. |
| Applicable instruments | ABI 7500, LightCycler 480, AGS4800, etc. |
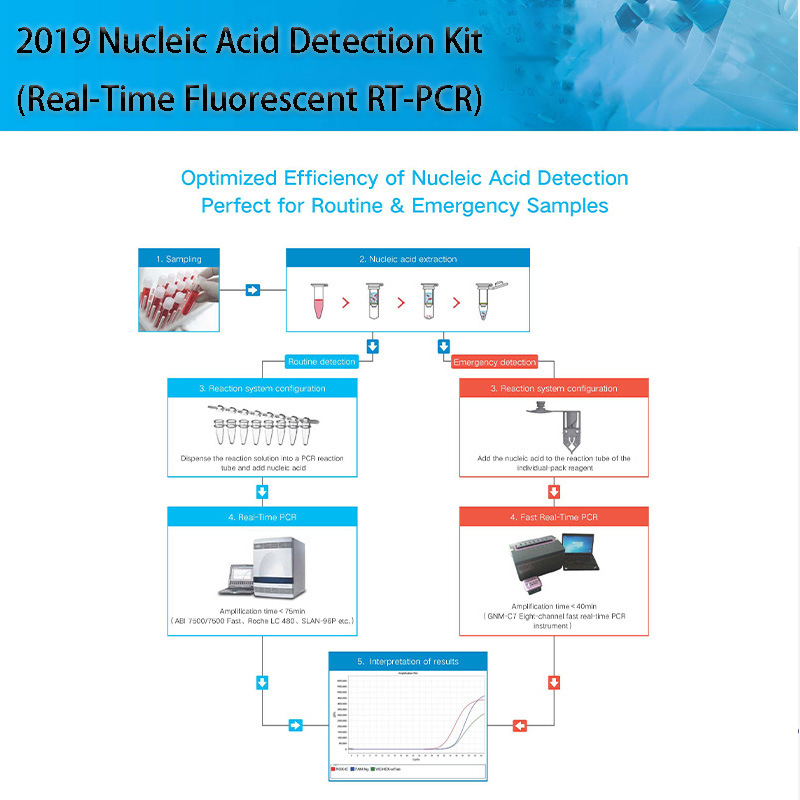
[Test Principle]
By multiplex PCR-fluorescent probe technology, combined with fast one-step RT-PCR technology, the kit uses ORF1ab and the specific conserved sequence of nucleocapsid protein N gene as target regions. With the designed dual-target gene detection primer probe, the kit works with a high-efficiency RT-PCR reaction system for detection. After the end of the reaction, the results will be determined through analysis of the cycle threshold (Ct) of each channel. Moreover, the kit is designed with human housekeeping gene RNaseP for monitoring sampling, extraction,specimen addition, amplification and other related processes as an internal standard to effectively prevent false positive and false negative results, so as to ensure the specificity and accuracy of the test results.
[Advantages and features]
1. Multiple fluorescence quantitative PCR, single tube reaction reduces the operation steps and improves the detection flux;
2. Premixed reaction liquid, more convenient operation;
3. Single tube preloading reagent to meet more laboratory requirements;
4. Rapid amplification, 40-75 minutes to complete the amplification reaction;
Laboratory tests showed that the amplification time of ABI7500 / slan-96 / LightCycler480 was < 75 min, and that of kinnomix C7 was < 40 min

Performance verification:
The novel Nucleic Acid detection kit(Real-Time Fluorescent RT-PCR)developed by bio-co., LTD., has passed the test of the Chinese center for disease control and prevention of viral diseases on February 1. According to the technical guidelines for laboratory testing of novel infected pneumonia (3rd edition), it is concluded that the fluorescence detection results of novel virus detection kit with two different targets are in good consistency with the laboratory detection methods. Moreover, hundreds of cases of clinical data were verified in many first-class hospitals in wuhan.
[Precautions]
1. Please read the entire Instructions carefully before using the kit.
2. This product does not contain nucleic acid extraction reagent, which needs to be prepared by the user.
Please refer to [Main Components] for details.
3. Please strictly follow the management practices of genetic amplification testing laboratories as issued by the industrial administrative department in charge.
4. This kit is an in vitro detection reagent, and the operating personnel must be professionally trained.
5. The entire detection process shall be strictly conducted in three areas, i.e.,Reagent Preparation Area, Specimen Preparation Area and Amplification Area.The instruments, equipment, consumables and work clothes used in each area shall be independent and dedicated.The workbench shall be cleaned immediately after the experiment and be disinfected.
6. Please use disposable dedicated centrifuge tubes, calibrated pipettes, tips with filter element, as well as disposable gloves that do not contain fluorescent materials. Gloves must be replaced frequently.
7. The components in the kit shall be used within the validity period. Do not mix reagents of different batches.
8. All reagents must be thawed completely, mixed well and centrifuged at 6,000rpm for a few seconds before use.
9. Instruments and equipment, such as operating tables, pipettes,centrifuges and PCR analyzer, shall be disinfected frequently with 10% sodium hypochlorite or 75% alcohol, UV or ozone.
10. Try to avoid air bubbles in the reaction tube. The tube cap must be tightened.
11. After the amplification is completed, take out the reaction tube, seal it in a special plastic bag, and discard it at the designated place.
12. The test specimens involved with this kit are considered to be infectious substances, and any operation and processing must meet the relevant requirements of General Biosafety Standard for Microbiological and
Biomedical Laboratories and Medical Waste Management Regulations released by the Ministry of Health of the P.R.C.
13. Test kit for professional use only
