Overview
Quick Details
Place of Origin:China
Power Source:Manual
Warranty:2 years
After-sale Service:Online technical support
Material:plastic, Antibody Igg/Igm Colloidal Gold Test
Shelf Life:2 years
Quality Certification:ce
Instrument classification:Class I
Safety standard:none
Type:Pathological Analysis Equipments
Certificate:CE/ ISO9001/ ISO13485
Specimen:swab/urine
Format:Strip, Cassette,
Accuracy:Over 99%
Size:2.5/3.0/4.0/5.5/6.0 mm
Package:Individual Foil Pouch
Usage:Professional Testing
Sensitivity:Antibody Igg/Igm Colloidal Gold Test
Packaging & Delivery
Packaging Details
1pc/box; 25pcs/box, 50 pcs/box, 100pcs/box, individual aluminium foil bag package for each piece product; OEM packing is available.
Lead Time :
1pc/box; 25pcs/box, 50 pcs/box, 100pcs/box, individual aluminium foil bag package for each piece product; OEM packing is available.
Lead Time :
| Quantity(Pieces) | 1 - 10000 | >10000 |
| Est. Time(days) | 30 | To be negotiated |
Product Description
Product Features | |
Product name | CE approved professional use Chlamydia rapid test |
Certificate | CE/ ISO9001/ ISO13485 |
Color | bule |
Specimen | swab/ urine |
size | strip(2.5mm,3.0mm, 3.5mm, 4.0mm) cassette(3mm, 4mm) or according to the customers' requirements |
Sensitivity | N/A |
Packing Details | strip Cassette 1pcs/foil bag, 50bags/mid-box, 40mid-boxes/carton; in bulk, 2000pcs/carton |
Shelf Life | 2years |
The Chlamydia Test is intended for in vitro diagnostic use in the rapid, qualitative detection of Chlamydia trachomatis directly from female endocervical swab and male urethral swab, in addition to ocular specimens from symptomatic patients. The test is intended for use as an aid in the diagnosis of Chlamydia infections. The test is a rapid qualitative immunoassay based on the immunochromatographic principle.
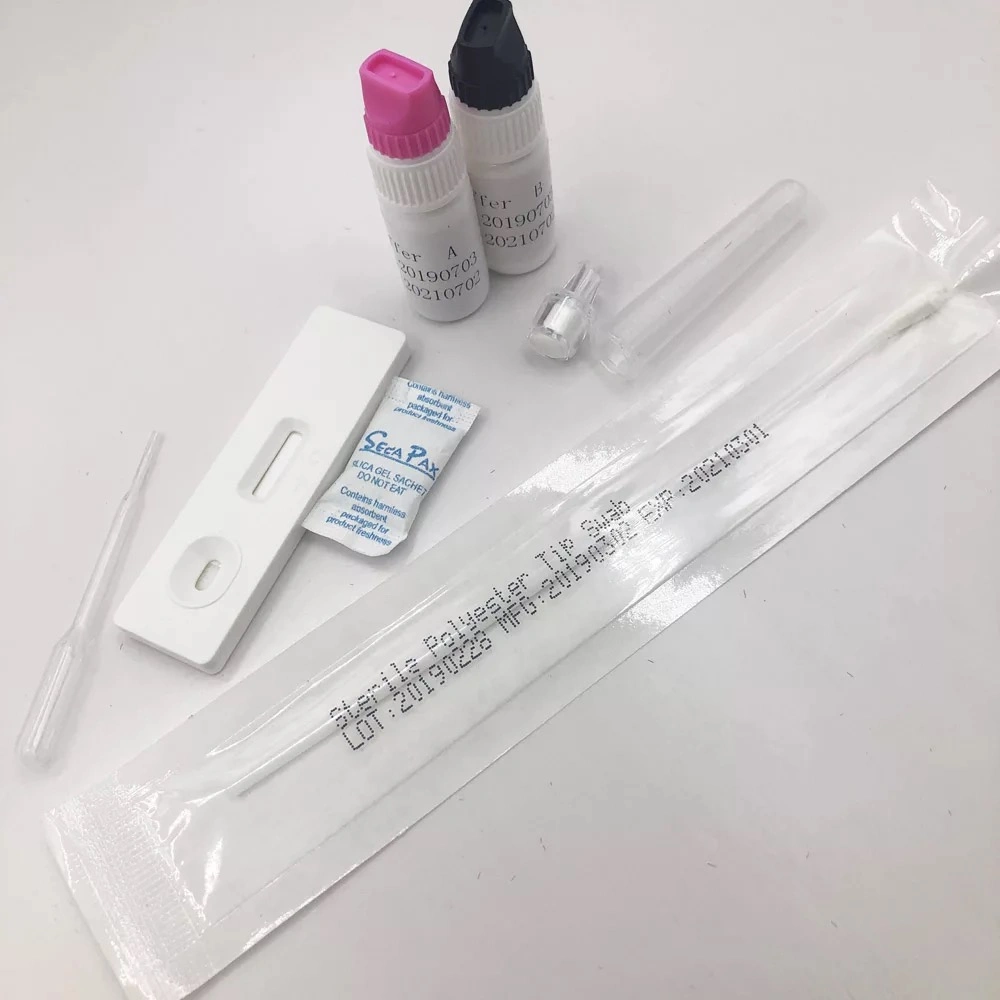

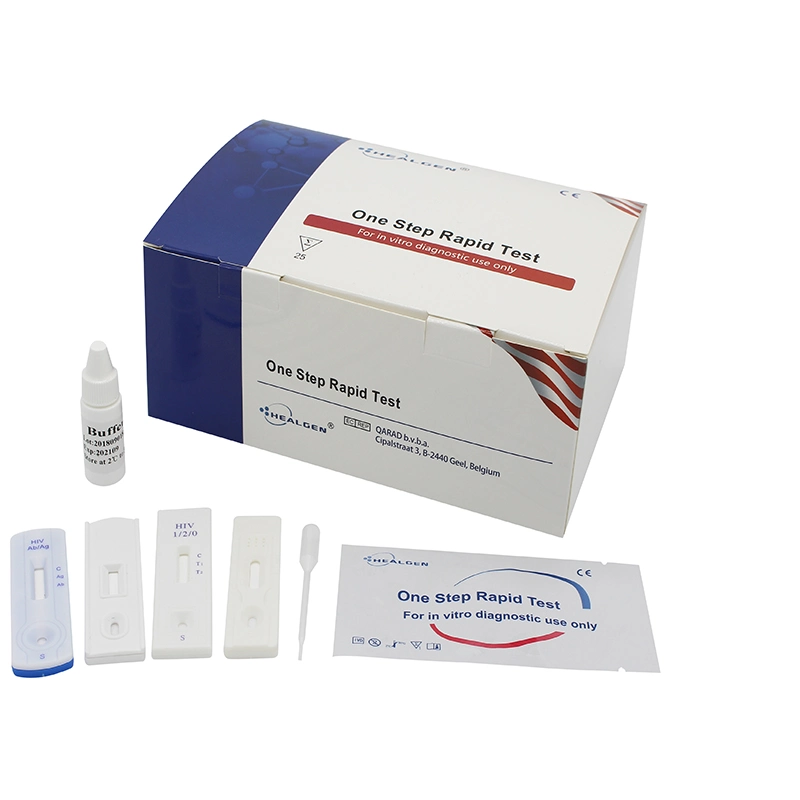



Test producer
1.one piece of test,
2. one pipette,
3.one swab,
4.one desiccant
5.reagent A+B.
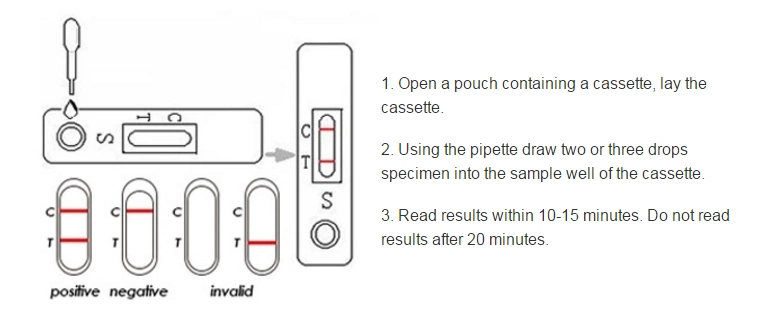
 Manufacturing Technique
Manufacturing Technique

2. one pipette,
3.one swab,
4.one desiccant
5.reagent A+B.

 Manufacturing Technique
Manufacturing Technique